Hydrochloride vs Base: What is the difference

David Blok | Posted on September 07, 2023
Introduction
Have you ever wondered why Amoxicillin comes in both Hydrochloride and Base forms and how this choice affects its efficacy in treating bacterial infections? If you’ve ever wondered why two medicines with the same ingredient can have distinct impacts, then you’re in for a treat. In today’s deep dive, we’re unraveling the fascinating contrast between Hydrochloride (Salt) and Base (Freebase) forms of APIs, a subject that holds sway over everything from manufacturing costs to patient outcomes.
Uncover why this seemingly minute difference can affect how a drug is stored, its bioavailability, and even its safety profile. Whether you’re a pharmaceutical professional seeking to optimize your product line, or a curious individual keen to understand what goes into your medicine—this comprehensive guide has something for everyone.
So strap in, as we decode the nuances and untangle the complexities that could very well revolutionize how you think about medicine.

Why the Different Forms of APIs Matter
Understanding the various forms of APIs isn’t just an academic exercise. It’s vital for drug development, efficacy, and even profitability. So, why should you care about whether an API is a Salt or Freebase?
Why Formulation Is Important
For basic drugs, the most common salt form is the hydrochloride; about 60% of all basic drug salt forms are hydrochlorides. The choice between Hydrochloride and Base can be pivotal, impacting the efficacy, shelf life, and even patient compliance. Each form has its pros and cons, making it crucial for manufacturers to weigh their options carefully.
Effect on Bioavailability
The form of the API can significantly influence how much of the drug is absorbed into the system, thus affecting its efficacy. Hydrochlorides often offer better bioavailability, making them the first choice for acute treatments. But it’s not a one-size-fits-all scenario, which leads us to our next point.
Breaking Down Chemical Structures
What Is Hydrochloride?
Hydrochloride is essentially the salt form of an API. By reacting the base form with hydrochloric acid, Hydrochloride often gains enhanced properties like increased solubility and better stability. For example, Cocaine Hydrochloride is far more stable than its base form. (Source)
What Is Base?
The base form, in contrast, is the API in its fundamental, unreacted state. These are usually less stable but can offer other advantages like ease of formulation and sometimes lower costs. (Source)

The Manufacturing Angle
Production of Hydrochloride Form
Opting for Hydrochloride typically means a longer, more complex production process. The addition of hydrochloric acid often requires additional steps like neutralization and further purification. The upside is a more stable, often more soluble end-product.
Production of Base Form
The base form is typically simpler to produce, requiring fewer steps and thus potentially lowering manufacturing costs. However, this can come at the cost of stability and solubility, making it less ideal for certain applications.

Stability & Storage
Hydrochloride Stability
When it comes to shelf-life, Hydrochloride usually takes the cake. Its inherent stability often translates to longer expiration dates, making it more appealing for stockpiling or long-term treatment plans. (Source)
Base Stability
The base form, in contrast, may degrade more rapidly. Its reduced shelf-life can limit its applications and make storage more challenging.
Solubility & Dissolution Rates
Solubility of Hydrochloride
Solubility isn’t just a scientific term; it’s a practical one. Higher solubility usually means quicker absorption into the bloodstream, making Hydrochloride the choice for treatments that require rapid onset. (Source)
Solubility of Base
The base form, while often less soluble, may offer advantages for extended-release formulations. This characteristic makes it ideal for medications that need to be released slowly over time.
Bioavailability & Efficacy
Impact of Hydrochloride
Because of its usually higher solubility, Hydrochloride forms often boast higher bioavailability. This can mean more effective treatments and could potentially reduce the required dosage, thereby lowering the risk of side effects.
Impact of Base
While the base form may offer lower bioavailability, it’s often used in slow-release pills, providing a longer-lasting but less immediate effect. In some cases, this may mean having to administer larger or more frequent doses to achieve the desired outcome.

Regulations and Certifications
Navigating the labyrinth of pharmaceutical regulations is no small feat. Here’s what you need to know about compliance in the context of API forms.
Hydrochloride
The Hydrochloride form is often under rigorous scrutiny. Manufacturers usually need to adhere to multiple sets of guidelines, including Good Manufacturing Practice (GMP), Certificate of Suitability to the monographs of the European Pharmacopoeia (CEP), and FDA approvals. This can add to the complexity and cost of production but ensures a higher quality end-product. (Source)
Base
Although still regulated, the base form may face fewer regulatory hurdles. This could make it a quicker route to market, but it’s crucial to ensure that quality isn’t compromised in the process.

Cost Implications
The financial burden of drug development can be staggering, and the choice between Hydrochloride and Base can add another layer to the budgetary considerations.
Financial Aspects of Hydrochloride
Higher production costs, stringent regulations, and extra testing requirements can make Hydrochloride a more expensive option. However, its superior stability and bioavailability could justify the additional cost.
Financial Aspects of Base
The base form, while often cheaper to produce, may end up requiring more substantial dosages to achieve the same effect as its Hydrochloride counterpart, thereby affecting its cost-effectiveness.

Safety & Side Effects
Understanding the safety profile of your API form is not just good practice; it’s a moral obligation.
Hydrochloride Safety Profile
Generally, Hydrochloride forms are well-tolerated. However, due to their enhanced solubility and bioavailability, there may be a higher risk of over-dosage or side effects.
Base Safety Profile
The base form may offer a softer impact in terms of side effects, but this could mean a requirement for higher doses, which carries its own risks and complications.
Applications & Use-Cases
From fast-acting antibiotics to slow-release mental health medication, the choice between Hydrochloride and Base has far-reaching implications.
Hydrochloride in Pharmaceuticals
Hydrochloride is often the go-to form for drugs that require rapid action, such as emergency pain relievers or fast-acting anti-allergens.
Base in Pharmaceuticals
The base form finds its niche in drugs requiring a slow-release mechanism, such as some types of antidepressants or extended-release pain management solutions.
Conclusion
The pharmaceutical world is brimming with complexities, and the choice between Hydrochloride and Base forms of APIs adds another layer to this intricate tapestry. Understanding the pros and cons of each can significantly impact everything from manufacturing to treatment efficacy and patient safety.
FAQ
Why are there different forms of APIs?
Different forms suit various applications, stability profiles, and bioavailability requirements.
What are hydrochlorides?
Hydrochlorides are salts formed from an organic base (usually an amine) and hydrochloric acid, enhancing the stability, solubility, and bioavailability of drugs. This conversion boosts absorption and therapeutic effectiveness, overcoming common formulation challenges. Key advantages include increased water solubility, improved stability against degradation, and easier manufacturing processes. Thus, using hydrochloride salts is a standard practice in optimizing medication performance.
Is one form generally better than the other?
Not necessarily. The “best” form depends on the specific requirements of the drug in question.
Do certifications matter when selecting a form?
Absolutely. Certifications like GMP, CEP, and FDA are crucial indicators of quality and compliance.
What role does solubility play in API forms?
Solubility directly influences how quickly a drug is absorbed into the bloodstream, which in turn affects its efficacy and potential side effects.
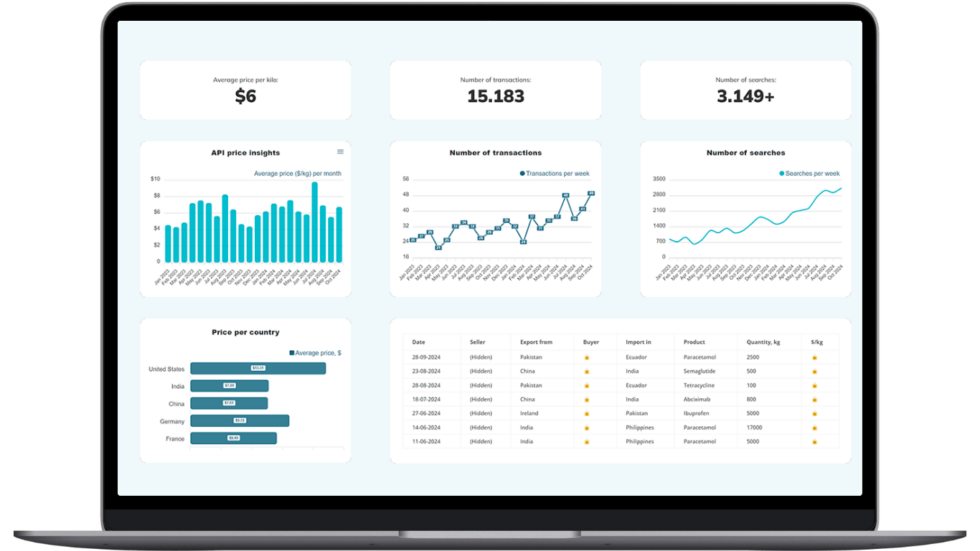
Make Smarter API Decisions with Data
Access exclusive insights on global API pricing, export/import transactions, competitor activities and market intelligence.






Check out all other blogs here!