
With the help of Accestra
How to apply for a China Drug Master File (cDMF)?
Overcome China’s DMF regulatory requirements easily by trusting cDMF regulatory affairs professionals who can open the Chinese market for your company.

Navigate China’s Pharma Landscape
What is a cDMF?
A cDMF, or China Drug Master File, is analogous to the Drug Master File (DMF) used in the United States but is specific to the regulatory requirements of China, the second-largest market in the world.
It is part of China’s regulatory framework for pharmaceutical products, managed by the National Medical Products Administration (NMPA), formerly known as the China Food and Drug Administration (CFDA).
From APIs to Excipients in China
The Path to cDMF
Like the U.S. DMF, the China DMF also encompasses three product scopes/categories and provides two registration pathways.
That cater to different components of pharmaceutical manufacturing:
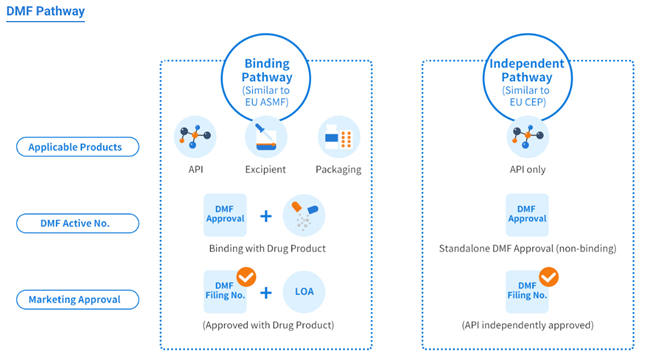
Product categories:
- Active pharmaceutical ingredients (APIs)
- Excipients
- Packaging materials
Registration Pathways:
- Binding review pathway (similar to the EU ASMF, covering all three product categories)
- Stand-alone review pathway (similar to the EU CEP, applicable to API products only)
Tool for Smoother Market Entry in China
Why do you need a cDMF?
Navigating the regulatory requirements in China’s pharmaceutical and medical device industry, especially for API suppliers and producers, can be overwhelming due to their intricate complexity.
These companies often face significant delays or rejections of their product registrations due to the constantly evolving compliance landscape, but that’s not their biggest challenge.
Despite aligning with international regulations like the International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use (ICH), China still requires a local legal entity to act as the registration representative for a non-Chinese company.
Failing to find a local representative with expertise in China’s Regulatory Affairs can result in costly non-compliance and regulatory missteps, such as:
- Delayed Product Launches
- Fines and Penalties
- Loss of Licensing
- Market Access Denial
- Financial losses and logistical challenges due to product recalls
You need a lot of time and energy to review this paperwork.
And remember that even minor oversights or mistakes can have significant consequences, such as financial and reputational damage.
The China DMF system allows pharmaceutical API manufacturers and suppliers to submit detailed and confidential information about facilities, processes, or materials used in drug manufacturing, processing, packaging, or storage.
This system ensures the NMPA has access to necessary data to assess compliance with regulatory standards without exposing proprietary information to the public or competitors.

Discover the World of cDMF Expertise
Expert cDMF Guidance with Accestra:
As we said before, having local expertise on your side is not only an advantage but a requirement for entering the China market.
With the help of experts, businesses can confidently navigate the regulatory landscape, avoid costly missteps and gain a competitive edge over other pharmaceutical businesses.
Why Accestra?
At Accestra Consulting, we specialize in the Chinese regulatory environment for pharmaceuticals, medical devices, and the food industry.
With a robust track record of over 100 successful product registrations, Accestra provides your company with tailored regulatory compliance and pharmacovigilance services that align with China’s National Medical Products Administration (NMPA) regulations, saving you a lot of time and energy.
A Team of Experts on the Chinese Regulatory Field
Accestra has a successful track record with over 100 product registrations covering but not limited to the following services:
Pharmaceutical Regulatory Advisory
- Pre-IND, Pre-NDA, Pre-BLA Meeting Application
- IND, CTA, BE Application
- NDA, BLA, ANDA Registration
- DMF Filing for APIs, Excipients, Packaging Materials
- Pharmacovigilance Services
- Regulatory Intelligence & Advisory
- China Market Access Roadmap Advisory
Medical Device Regulatory Advisory
- Medical Device Classification Determination
- Medical Device Filing for Class I Products
- Medical Device Registration for Class II & III Products
- Medical Device Raw Materials/Key Components Mater File Filing
- Regulatory Intelligence & Advisory
Food Regulatory Advisory
- GACC China Customs Registration
- China Food Ingredients Check
- China Food Labelling Compliance
- Food Supplement Registration
- Regulatory Intelligence & Advisory
Trusted by:















Reduce risks, enhance speed to market, and ensure sustained compliance
Make Accestra your regulatory partner to succeed in the Chinese market with ease with 0 worries.

Accestra’s Step-by-Step Dossier Preparation and Submission
How to Apply for a Chinese DMF?
As we have seen, market approval is required for every overseas pharmaceutical enterprise to sell its products in China.
Preparing the registration dossier according to China CDE’s format and content requirements can be difficult and time-consuming, plus there can be many submission deadlines to account for.
ACCESTRA takes the pressure off of you with a simple step-by-step preparation process:
- Regular affairs experts provide a registration strategy to smooth your entry process.
- Pharmaceutical experts ensure accurate translation of your dossier into Chinese.
- Create a CDE submission timeline for market approval.
- Extended DMF, NDA and ANDA preparation for a successful submission.
- Meet the most recent requirements of China CDE.
- Prepare your dossier in electronic CTD format as per CDE’s requirements.
For us, extended preparation means a high-quality dossier with no delay in submission.
Because we are in close contact with every Chinese regulatory agency for product registration, we understand dossier requirements comprehensively, so you don’t have to.
Let us act as your Chinese agent for product registration and meet every requirement.
