Press Release
Shilpa Medicare Limited

August 29, 2024
Shilpa Medicare Limited Announces Multiple Milestones in Innovation and Global Approvals
Greetings from Shilpa Medicare Limited…!
Shilpa Medicare Limited is proud to share a series of groundbreaking achievements that reinforce our commitment to innovation and excellence in healthcare.
Successful Completion of Phase 1 Clinical Trial for sRbumin® (rHA 20%)
We are delighted to announce the successful completion of Phase 1 clinical trials for our flagship product, sRbumin® – recombinant human albumin (rHA 20%). The positive results from the trial underscore rHA’s potential as a viable alternative to plasma-derived human serum albumin, addressing a critical gap in global healthcare.
EMA Positive Feedback for Aflibercept Injection
Shilpa Biologicals Private Limited, a subsidiary of Shilpa Medicare, has received positive scientific advice from the European Medicines Agency (EMA) for its Aflibercept Injection (2mg/0.05ml). This biosimilar to Eylea is a significant step forward in treating conditions like neovascular (wet) age-related macular degeneration and diabetic macular edema.
Launch of Rivaroxaban Oral Dispersible Film
We are proud to announce the development of a novel dosage form of Rivaroxaban in Oral Dispersible Film, a first of its kind globally. This innovative form, which does not require water for administration, offers improved patient compliance, particularly for pediatric, geriatric, and psychotic patients.
ANVISA Brazil Approval for Shilpa Pharma Lifesciences
Shilpa Pharma Lifesciences has received ANVISA Brazil approval for its world-class manufacturing facility, marking a significant milestone in our global expansion. This facility, equipped with state-of-the-art isolator technology, is set to produce high-quality active pharmaceutical ingredients (APIs) for oncology and other therapeutic areas.
Launch of Pemetrexed Injection RTU in the US Market
Shilpa Medicare Limited has launched the world’s first ready-to-use Pemetrexed Injection formulation in the US market, already granted a permanent J-code (J9324) by the U.S. Centers for Medicare & Medicaid Services. This novel RTU formulation offers significant benefits in terms of convenience and storage for oncology treatments.
European Approval for Varenicline Tablets (0.5mg and 1mg)
We are pleased to inform that Shilpa Medicare has received European approval for Varenicline Tablets, used for smoking cessation. This achievement allows us to contribute to global public health by providing high-quality alternatives for smoking cessation.
USP Recognition for API Standards
Shilpa Medicare Limited is honored to have received a Certificate of Appreciation from the United States Pharmacopeia (USP) for our efforts and contributions to the standard-setting process. This recognition reflects our unwavering commitment to maintaining the highest quality standards in the pharmaceutical industry. Our APIs and standards continue to support global health initiatives, reinforcing our role as a trusted partner in healthcare.
Introduction of AI-Powered Drug Discovery Platform – NETRA AI
In our ongoing pursuit of innovation, we are excited to introduce “NETRA AI,” an advanced artificial intelligence-powered drug discovery platform. NETRA AI is designed to revolutionize the drug development process by significantly reducing R&D timelines and costs, while improving hit rates and optimizing resource utilization. This platform represents a significant leap forward in how pharmaceutical companies can approach drug discovery, ensuring that they remain competitive in a rapidly evolving market.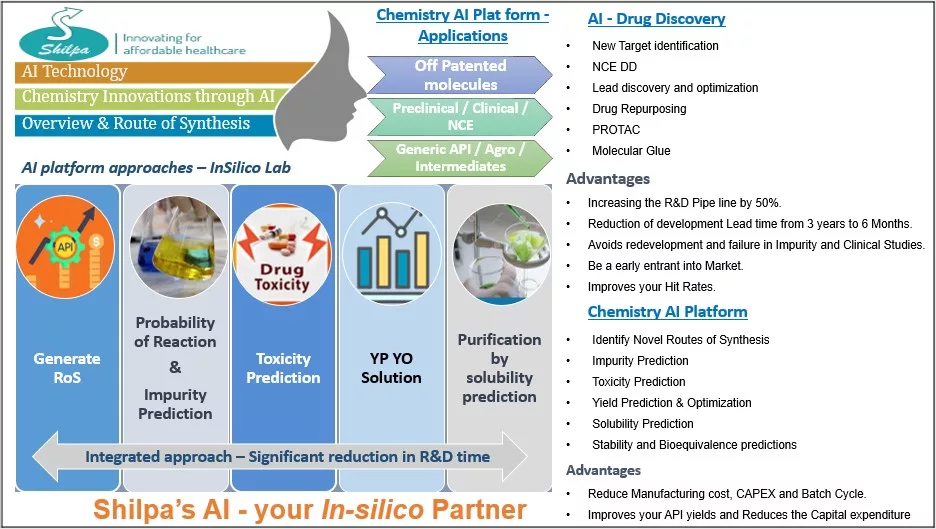
Shilpa Medicare’s Comprehensive Capabilities and Global Reach
With over 30 years in the pharmaceutical industry, Shilpa Medicare Limited continues to expand its global footprint, offering a comprehensive portfolio including APIs, formulations, biologics, transdermal patches, and oral films. Our vertically integrated operations, supported by nine manufacturing facilities, five R&D centers, and four analytical labs, allow us to deliver high-quality products efficiently. With over 250 patents granted and collaborations with 75+ global partners, Shilpa Medicare is positioned as a leader in pharmaceutical innovation.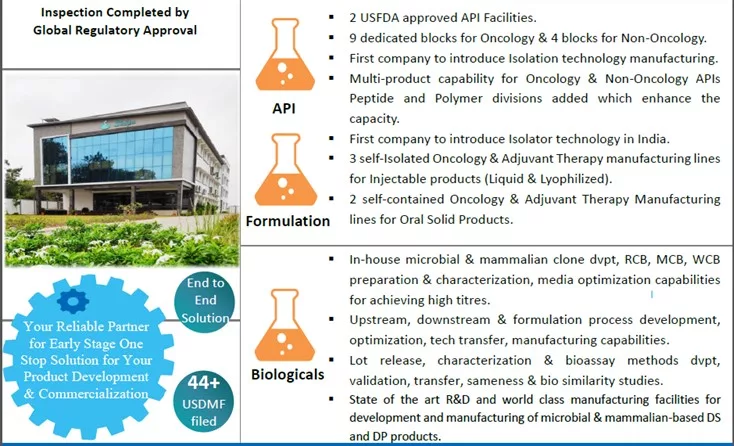
About Shilpa Medicare Limited
Shilpa Medicare Limited, a leader in the global pharmaceutical industry for over 30 years, focuses on “Innovating for Affordable Healthcare.” Our vertically integrated operations encompass the full pharmaceutical value chain, including APIs, finished formulations, biologics, transdermal patches, and oral films.
With nine manufacturing plants, five R&D centers, and four analytical labs, we adhere to the highest quality standards, certified by global regulatory agencies such as the USFDA, EMA, WHO-GMP, and ANVISA Brazil.
Shilpa Medicare is committed to improving patient outcomes through high-quality, affordable medicines, supported by a team of over 4,000 professionals. We aim to create a healthier, sustainable future through innovative products, partnerships, and social responsibility.
Contact
For further information or partnership opportunities, please contact us at:
- Madhav S: Madhav@vbshilpa.com
- Keshav S: Keshav@vbshilpa.com
- Hanumanth R: Hanumanth@vbshilpa.com





