How to Apply for a GMP Certificate?
Everything you need to know about the procedure
Priya Bhat | Posted on June 7, 2023
Introduction
In today’s globalized world, where consumers are increasingly concerned about the safety and quality of products, obtaining a Good Manufacturing Practice (GMP) certificate has become essential for businesses in the pharmaceutical, food, and cosmetic industries. A GMP certificate assures that a company follows a set of stringent quality standards, ensuring the production of safe and reliable products.
However, the process of applying for a GMP certificate can be complex and overwhelming. In this comprehensive guide, we will walk you through the step-by-step process of applying for a GMP certificate, providing valuable insights and tips along the way.
Step 1: Understanding GMP
Understanding Good Manufacturing Practices (GMP) is like unlocking the secret to producing safe and high-quality products. GMP serves as a set of guidelines and regulations that ensure consistent quality throughout the manufacturing process.
Think of it as a quality compass that guides businesses in the pharmaceutical, food, and cosmetic industries. By following GMP, companies can establish rigorous standards for facility design, personnel training, documentation, quality control, and hygiene practices. GMP is all about meticulous attention to detail, traceability, and adherence to regulatory requirements. It encompasses everything from raw material sourcing to product packaging and labeling.
By understanding and implementing GMP, businesses can gain the trust of consumers, enhance market access, and stay compliant with industry standards. Think of it as the cornerstone of producing products that meet the highest standards of safety, efficacy, and reliability. So, embrace GMP, and let it be your guiding light on the path to manufacturing excellence.
Step 2: Conducting a GMP Gap Analysis
Conducting a GMP gap analysis is like embarking on an exciting treasure hunt to uncover areas for improvement in your manufacturing practices. Think of it as a comprehensive check-up that assesses your current processes against GMP requirements.
By conducting this analysis, you can identify any gaps or deviations from the desired standards. It’s like shining a spotlight on potential opportunities for enhancement and ensuring regulatory compliance. Through a systematic evaluation of facility design, personnel training, documentation, quality control, and hygiene practices, you can pinpoint areas where adjustments are needed. It’s not about finding faults but rather about embracing a proactive approach to continuous improvement.
By addressing the gaps identified during the analysis, you can elevate your manufacturing processes to meet and exceed GMP standards. So, grab your detective hat, embark on the adventure, and unleash the potential to enhance your operations, ensuring the production of safe and high-quality products that surpass customer expectations.
Step 3: Developing a GMP Implementation Plan
Developing a GMP implementation plan is like charting a course for success in manufacturing excellence. Think of it as a roadmap that outlines the steps you need to take to ensure compliance with Good Manufacturing Practices (GMP).
It’s your chance to put all the pieces together and create a systematic approach to quality and safety. By developing this plan, you establish clear objectives, assign responsibilities, and define timelines for implementation. It’s like building a strong foundation for your operations. From GMP certified facility design to personnel training, documentation, quality control, and hygiene practices, every aspect is carefully considered and incorporated into the plan. It’s all about creating a culture of quality and a commitment to following regulatory requirements.
By following your GMP implementation plan, you can ensure consistency, traceability, and the production of safe and reliable products. So, gear up, embrace the plan, and set sail on a journey toward manufacturing excellence and customer satisfaction.
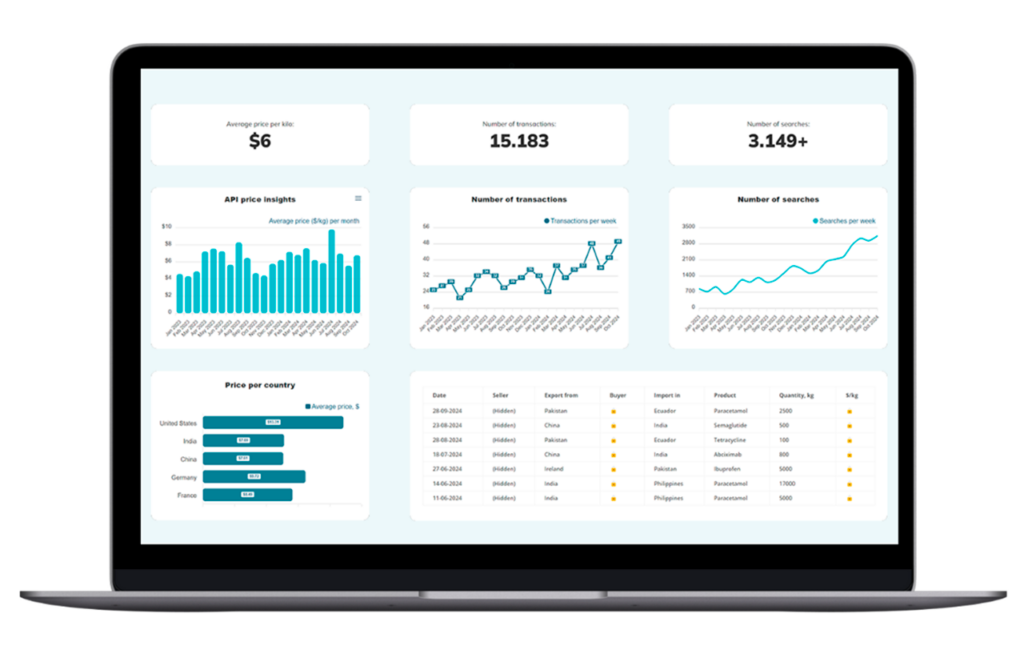
Want to be sure you’re paying a fair price for your APIs? Now it’s easy.
Pharmaoffer’s Trade Data service gives you an overview of the latest average selling prices, transaction volumes, and tracking search data across the globe for thousands of APIs.
Simplify your sourcing process by accessing current market insights and detailed transaction histories that will give your business a competitive edge.
Check it out today and make smarter sourcing decisions!
Step 4: Documentation and Standard Operating Procedures
Documentation and Standard Operating Procedures (SOPs) are like the superhero sidekicks of Good Manufacturing Practices (GMP). They ensure consistency, traceability, and efficiency in your manufacturing processes. Think of documentation as the secret language that captures all the essential details of your operations. From ingredient specifications to manufacturing instructions, it’s your comprehensive record-keeper.
SOPs, on the other hand, are like your trusty guidebooks, providing step-by-step instructions for every task and process. They ensure that everyone in your organization follows the same standardized procedures. Together, documentation and SOPs are the backbone of quality control, helping you maintain compliance with regulatory requirements.
By documenting your processes and implementing clear SOPs, you create a reliable framework that promotes safety, quality, and consistency.
Step 5: Preparing for GMP Certification Audit
Preparing for a GMP certification audit is like getting ready for a grand performance on the regulatory stage. Think of it as a chance to showcase your commitment to Good Manufacturing Practices (GMP) and demonstrate that your operations meet the highest standards. It’s a moment to shine and prove that you’re following the rules.
To prepare, you need to gather your documentation, polish your processes, and ensure that everything is in top shape. It’s like fine-tuning the instruments before a symphony performance. Review your standard operating procedures, quality control measures, and training records. Conduct mock audits to identify any potential gaps and address them proactively. It’s all about being thorough and proactive.
When the audit day arrives, be confident in your preparations and welcome the auditors as partners in your pursuit of quality and compliance. By preparing diligently, you’ll not only pass the certification audit but also gain peace of mind, knowing that you’ve built a solid foundation for the production of safe and reliable products. So, break a leg and show the world your commitment to GMP excellence!
Conclusion
Obtaining a GMP certificate is a significant milestone for businesses in the pharmaceutical, food, and cosmetic industries. In this guide, we have explored the step-by-step process of applying for a GMP certificate, covering essential aspects such as conducting a gap analysis, developing an implementation plan, establishing a QMS, and preparing for the certification audit. By following these guidelines and maintaining a commitment to continuous improvement, businesses can demonstrate their dedication to producing safe and high-quality products, gaining a competitive edge in the marketplace.
Remember, obtaining a GMP certificate is not a one-time achievement but an ongoing commitment to maintaining compliance with GMP standards. Stay informed about updates and regulatory changes, and continuously strive for excellence in your manufacturing practices.
FAQ