David Blok | Posted on October 3, 2020
What are Residual solvents and Elemental impurities?
Residual solvents and elemental impurities are two pharmaceutical guidelines that took effect relatively recently. The current revision of the residual solvents guideline was carried into effect as of June 2017, and the current revision of the elemental impurities guideline as of January 2018. In this article, we’ll explain them in a shortened version without using too many technical terms.
Residual solvents
During the production of pharmaceuticals (APIs), certain solvents can be used in the production- or purification process. There are dozens of different solvents, but for example, commonly used solvents are ethanol and acetone.
Solvents can be used to get a greater yield out of the raw material, to ensure a certain purity, or to achieve characteristics such as the crystallized form of raw material.
However, only some of these solvents may be removed entirely during manufacturing. If they are adequately removed, these solvents can protect the quality and even the safety of a drug product.
As this could form a risk to the end-user, specific guidelines were set up to ensure the safe use of solvents. They’re called the ICH Q3D and USP <467> guidelines and classify solvents in three different classes based on (amongst other things): their toxicity, carcinogenicity, and how dangerous they are to the environment,
In short, they are:
Class 1 solvents: Solvents are not to be used.
Class 2 solvents: Solvents only to be used within set limits.
Class 3 solvents: Solvents with low toxic potential, no set limits.
Contrary to many pharmaceutical guidelines, these ICH and USP guidelines are harmonized.
The only difference is that the ICH Q3C guideline applies to new drug products only, and the USP guideline is also required for pre-existing drug products.
Elemental Impurities
Cd, Hg, Mo, Ni, and Pb.
Perhaps you know what these letters mean by heart; maybeadequately removed, these solvents can protect the quality and you recognize them from your science class back when you were at school, or perhaps you need to learn, maybe inadequately, what they stand for.
Either way, they are relevant in the pharmaceutical industry, as they are a couple of the 24 elements that are listed in the “elemental impurities” guidelines: ICH Q3D and USP <232> & <233>.
Elemental impurities are traces of metals that can be present in drug products. Metals can be added intentionally during production to act as a catalyst. Still, they can also be released due to the production process, which can only sometimes be avoided.
If the amount of these traces is too high, it could have adverse effects such as toxicity and/or lessened effectiveness of the drug, amongst other things.
To prevent this from happening and to have control over elemental impurities, guidelines with certain limits were set up for 24 different elements.
The guideline divides the elements into different classes and provides analytical procedures for measuring these impurities. The toxicity limits of these elements are defined as maximum PDE (permitted daily exposure), and the different classes the elements are placed in have different ranges of PDE. Class number 1 has the elements with the lowest PDE and thus contains the most dangerous elements.
The 24 elements that are defined in the guideline
- Class 1
As – Arsenic
Cd – Cadmium
Hg – Mercury (hydrargyrum)
Pb – Lead (plumbum) - Class 2A
Co – Cobalt
Ni – Nickel
V – Vanadium - Class 2B
Ag – Silver (argentum)
Au – Gold (aurum)
Ir – Iridium
Os – Osmium
Pd – Palladium
Pt – Platinum
Rh – Rhodium
Ru – Ruthenium
Se – Selenium
Tl – Thallium - Class 3
Ba – Barium
Cr – Chromium
Cu – Copper
Li – Lithium
Mo – Molybdenum
Sb – Antimony (stibium)
Sn – Tin (stannum)
References
We hope this blog has taught you something about these guidelines. If you want more in-depth information about these guidelines, head to the ICH website for their Q3C or Q3D guidelines.
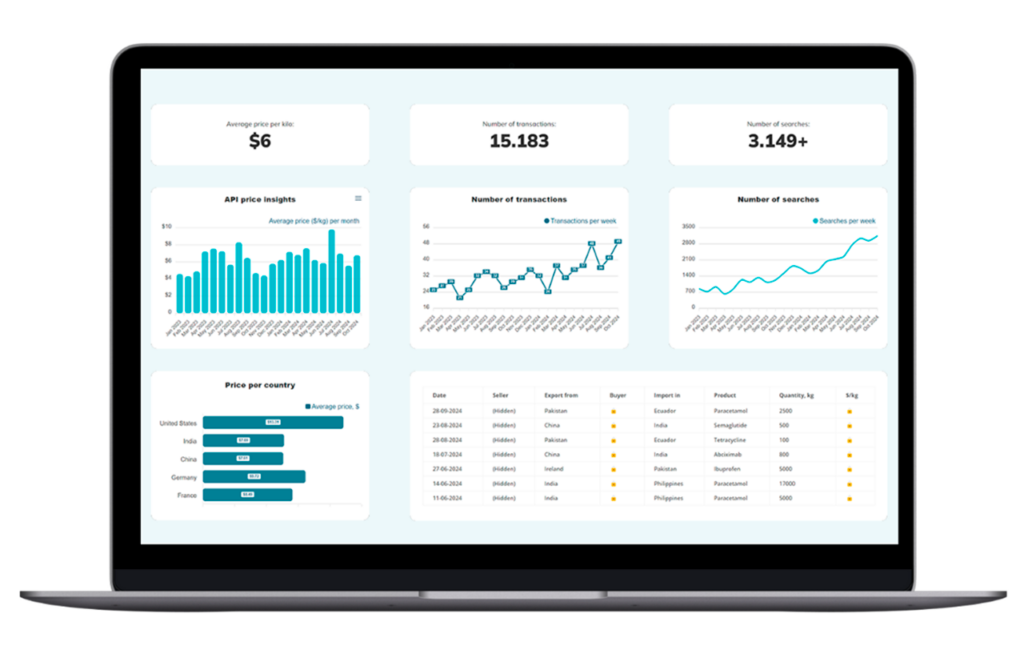
Want to be sure you’re paying a fair price for your APIs? Now it’s easy.
Pharmaoffer’s Trade Data service gives you an overview of the latest average selling prices, transaction volumes, and tracking search data across the globe for thousands of APIs.
Simplify your sourcing process by accessing current market insights and detailed transaction histories that will give your business a competitive edge.
Check it out today and make smarter sourcing decisions!