CMOs and CDMOs Explained:
Why They Matter in Pharma
Ammar Badwy
Posted on November 11, 2022 | Updated on 18 July, 2024
Introduction: The Role of CMOs in Medicine Production
The Challenge for Medical Researchers
Envision dedicating your entire career to unraveling the complexities of one single disease. This is the daily life of countless medical researchers who spend years (sometimes their whole career!) in a lab, studying diseases to advance patient care. Each day, they navigate a labyrinth of biochemical processes, driven by the hope of discovering a treatment that could alter the course of the disease.
However, thankfully, one day, a breakthrough arrives. After years of false starts and near misses, a team identified a compound showing trial promise. The joy is palpable, but the journey doesn’t end here.
The challenge now is to transform this laboratory success into a widely available treatment.
This is where Contract Manufacturing Organizations (CMOs) and Contract Development and Manufacturing Organizations (CDMOs) come into play. They are the bridge of groundbreaking research, transforming it into a new treatment source. CMOs and CDMOs specialize in scaling up production from the experimental stages carried out in research labs to the mass production needed to meet global demand. They navigate the complex regulatory landscapes, ensure quality control, and manage the logistics of distribution, providing a sense of reassurance that the new treatment can be safely and efficiently delivered to those in need around the world.
Who are CMOs?
Big Pharma vs. CMOs
You are probably familiar with the big names in the pharmaceutical industry, such as Pfizer, Johnson and Johnson and Roche. But have you ever heard of Lonza, Glatt and Catalent? These are part of the 110 billion dollar drug CMO market that is rapidly expanding. You may not have heard them because CMOs are the hidden champions of pharma, so to speak.
Although they manufacture the drugs themselves, their names are not on the label. Why is that, you may ask? Well, because they don’t own the property rights for the drugs. They get hired by small start-ups, research organizations and even big pharma companies, like J&J, to manufacture the drugs for them. But not all CMOs are the same!
What different types of CMOs are there?
Commonalities and Differences
CMO, CRO, CDMO. Different letter combinations, but all have one thing in common: they provide services to other companies in the pharmaceutical industry on a contract basis. In a way, you can divide them by the phases of product development. There is a lot of overlap, but the process starts with a Contract Research Organization (CRO), sometimes called a Clinical Research Organization. It’s an organization that takes the lead in managing trials and is responsible for complex medical testing procedures for its clients.
Once the product successfully passes that early stage, a CDMO, Contract Development & Manufacturing Organization, supports its clients during the drug development phase. Then, there is the CMO, a Contract Manufacturing Organization specializing in producing an already commercialized drug. They can do parts of the development, but the main focus is on getting the production done.
This is just one way of dividing them, but you can also divide them by product groups, such as APIs, vaccines or ointments. Another way is to separate a CDMO by its focus on discovering new APIs and chemical entities or on producing a generic version of a drug after the patent on it has expired.
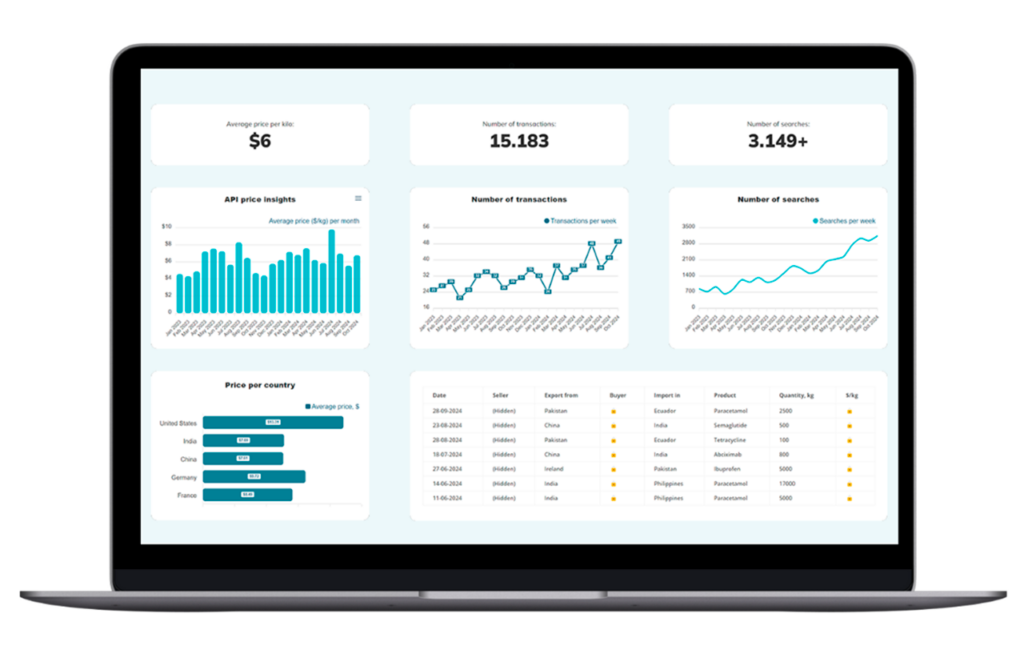
Want to be sure you’re paying a fair price for your APIs? Now it’s easy.
Pharmaoffer’s Trade Data service gives you an overview of the latest average selling prices, transaction volumes, and tracking search data across the globe for thousands of APIs.
Simplify your sourcing process by accessing current market insights and detailed transaction histories that will give your business a competitive edge.
Check it out today and make smarter sourcing decisions!
How to find the right CMO for your project?
Pre-Evaluation Steps
Choosing the proper Contract Manufacturing Organization (CMO) is crucial in pharmaceutical development. It requires a thorough pre-evaluation and understanding of what each CMO can offer. Kurt In Albon, who has dedicated his professional life to working at Lonza—one of the largest CMOs globally—shares his insights as the Director and global head of information quality.
“One of the first steps I recommend is visiting the FDA website to check for any Warning Letters, which can give you a good sense of a CMO’s quality culture,” he advises.
Additionally, conducting an audit is a common practice among pharmaceutical companies to gauge a CMO’s capabilities directly.
CMOs offer various services, including formulation, blending, coating, analytical testing, packaging, sterilization, and shipping. To navigate through these options effectively:
Assess Experience and Expertise: Start by confirming that the CMO has the necessary experience and expertise to handle your specific product. This ensures they can meet your project’s unique demands.
Facility Inspection: Visit their facilities to inspect the equipment, review procedures, and meet the staff. This on-site evaluation is crucial in assessing whether their operations align with your quality standards and regulatory requirements.
Gather External Insights: Engage with your professional network to gather first-hand accounts of experiences with the CMO. Reaching out directly to their past or current clients can provide invaluable insights into their service quality and reliability.
By thoroughly vetting potential CMOs using these steps, you can select a partner that meets your production needs and upholds the integrity and quality required in pharmaceutical manufacturing.
The Role of Communication and Partnership
Finally, yet most importantly- can you see yourself dealing with those people for many years to come? Sometimes, it comes down to pure gut feeling. Good communication, mutual understanding and support are vital for this partnership.
Philippe Tschopp is Head of Business Development at Glatt, a large CDMO. He emphasizes that there needs to be a good connection between both parties.
I always say that we need to be comfortable because the comfort level on both ends is essential. Because, in the end, in a successful project, you stick together for many many years.
How do CDMOs make money?
Pricing Strategies
Pricing strategies depend on what the CDMO makes and the company type. Some companies will charge per batch or weight, such as a price per kg. But you can also think of constructions where rent is paid for the manufacturing site, a fee for development cost and quality services. When a CRO is involved in discovering the product, it can also charge a fixed percentage of the profit.
The potential for growth in the CDMO industry is immense. Sometimes, contracts are so substantial that a CMO will even build an entire new plant dedicated to just that client. This growth potential is a promising sign for the industry and those involved in it.
What does the future hold for the CMOs?
The Future of CMOs: Market Predictions and Evolving Trends
The Contract Manufacturing Organization (CMO) market is on a robust growth trajectory. Analysts predict a significant expansion, with the CMO pharmaceutical market projected to increase by almost 50% by 2025, reaching a global value of $162.1 billion USD. This surge is largely fueled by the rapid advancements in AI-driven drug discovery techniques, which have revolutionized the speed at which new drug entities reach the market while substantially reducing research costs. As these technologies continue to evolve, there will be a burgeoning demand for new manufacturing capacities to keep pace with the accelerated drug development timelines.
Moreover, the broader Contract Development and Manufacturing Organization (CDMO) market is also witnessing substantial growth. Estimated at USD 146.0 billion in 2023, the CDMO market is expected to grow at a Compound Annual Growth Rate (CAGR) of 7.2% from 2024 to 2030. This growth is driven by an increasing demand for novel therapies, positioning CDMOs as critical players in the pharmaceutical landscape. For more detailed insights on the CDMO market trends and predictions, you can read our related blog post on the CDMO market trends and top CDMO companies.
As highlighted in the trend reports from NFS.org, 2024 is poised to introduce transformative trends in the CDMO sector. The industry is shifting towards models of equal partnership, where CDMOs and pharmaceutical companies are viewed as integral collaborators. This evolution marks a departure from traditional transactional outsourcing, emphasizing a more integrated and collaborative approach. The push for “one-stop shop” solutions is gaining momentum, streamlining the process from drug development to market by offering comprehensive, end-to-end services that enhance efficiency.
The focus is also shifting towards collaborative risk management, moving away from penalty-based models to those that reward achievements. This fosters a deeper level of trust and accountability in CDMO pharma partnerships. Additionally, the sector is experiencing significant reshaping due to merger and acquisition activities, with major transactions indicating shifts in market dynamics and creating both opportunities and challenges.
As CDMOs continue to expand globally, there’s a growing necessity to diversify operations and adapt to multiple regulatory environments and stakeholder expectations worldwide. This trend highlights the importance of geographic and operational diversification in managing the global pharmaceutical supply chain effectively.
In conclusion
When contracting a CMO or CDMO, you will have the peace of mind to get the necessary support with the regulatory aspects. You will have to worry much less about scaling up, establishing GMP-compliant processes, and getting the proper regulatory approvals. CDMOs are highly experienced in creating compliant and effective manufacturing processes and will have your back.
On the other hand, you become dependent on another company. You have to ensure that your contracts cover everything, such as intellectual property, quality, costs and data security.
It’s indisputable that CMOs play a crucial role in the pharmaceutical industry. Their services are not just beneficial but essential, and their presence is set to continue shaping the industry in the future.