What is a CRO?
Contract Research Organization
Alexander Doroshenko | Posted on September 13, 2023
- Introduction
- The Basics of CROs
- Types of CROs
- The Role of CROs in Clinical Trials
- Benefits and Challenges
- How to Choose a CRO
- Conclusion
- FAQs
Introduction
Ever wondered who’s behind the scenes, ensuring that the medicines you take are safe and effective? Meet the Contract Research Organizations (CROs), the unsung heroes of the pharmaceutical world. In this article, we’ll dive deep into what CROs are, why they’re indispensable, and how they operate.
So, are you ready to go behind the scenes?
The Basics of CROs
What Do CROs Do?
Pharma Contract Research Organizations, commonly known as CROs are the backbone of the pharmaceutical and biotechnological industries. They offer a plethora of services that span the entire drug development lifecycle. From the initial stages of research and development (R&D) to pre-clinical trials, clinical trials, and even post-market surveillance, CRO pharmaceutical services do it all. Imagine them as the Swiss Army knife in a researcher’s multi-functional, reliable, and absolutely indispensable toolkit.
Services Offered
- Drug Discovery: This is where it all begins. CROs assist in identifying potential drug candidates through various biochemical assays and screenings.
Curious about how new APIs are discovered? Read This is how new APIs are discovered.
- Pre-Clinical Trials: Before a drug can be tested on humans, it undergoes rigorous testing in the lab. CROs manage these pre-clinical studies, ensuring they meet all regulatory guidelines.
- Clinical Trials: This is the phase most people are familiar with. CROs manage drug testing on human subjects, collecting and analyzing data to determine efficacy and safety.
- Regulatory Submissions: Once a drug has proven effective and safe, it needs governmental approval. CROs help in the preparation and submission of regulatory documents.
- Post-Market Surveillance: Even after a drug hits the market, it must be monitored for long-term effects, another area where CROs contribute.
Why Are CROs Important?
The pharmaceutical industry is a behemoth, with global spending expected to reach $1.9 trillion by 2027. But did you know that the average cost to develop a new prescription drug is a staggering $2.6 billion? And that’s not even considering the time factor—it can take up to 12 years for a drug to go from the lab to the pharmacy shelf.
This is where CROs come in. They accelerate the drug development process, making it more efficient and cost-effective. By outsourcing specific tasks to experts, pharmaceutical companies can focus on what they do best, whether it’s R&D, marketing, or distribution.
Cost Savings
One of the most significant advantages of working with a CRO is cost savings. Running in-house clinical trials can be prohibitively expensive, requiring specialized staff, equipment, and facilities. CROs, with their expertise and resources, can often perform the same tasks at a fraction of the cost.
Global Reach
In today’s globalized world, clinical trials often span multiple countries, if not continents. CROs have the global reach and local knowledge to manage such complex, multi-site trials, ensuring they meet the regulatory requirements of each jurisdiction.
Expertise and Specialization
Another reason CROs are invaluable is their specialized expertise. Pharmaceutical companies often have broad focuses, from R&D to marketing. On the other hand, CROs specialize in specific aspects of drug development, bringing a level of expertise that’s hard to match.
Risk Mitigation
Drug development is fraught with risks, both financial and clinical. CROs help mitigate these risks by ensuring the trials are conducted ethically and scientifically, adhering to all regulatory guidelines. This not only safeguards the patients but also protects the pharmaceutical companies from potential legal issues.
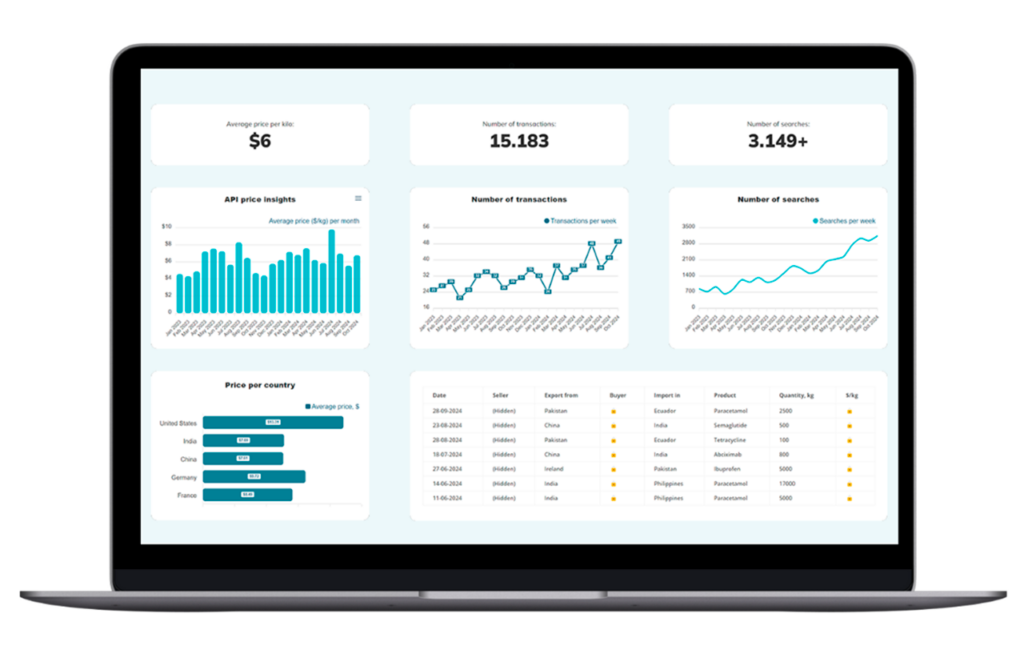
Want to be sure you’re paying a fair price for your APIs? Now it’s easy.
Pharmaoffer’s Trade Data service gives you an overview of the latest average selling prices, transaction volumes, and tracking search data across the globe for thousands of APIs.
Simplify your sourcing process by accessing current market insights and detailed transaction histories that will give your business a competitive edge.
Check it out today and make smarter sourcing decisions!
Types of CROs
Full-service CROs
When you think of a one-stop shop for all your drug development needs, you think of a full-service CRO. These organizations offer end-to-end solutions, covering everything from pre-clinical trials to post-market surveillance.
Advantages
- Simplicity: Working with a full-service CRO simplifies the drug development process, as you have a single point of contact for all stages.
- Consistency: With the same team overseeing the entire process, there’s greater consistency in data collection and analysis.
- Cost-Efficiency: Bundling services often leads to cost savings, making full-service CROs an economical choice for many companies.
Specialized CROs
Not all drug development projects require a full range of services. Sometimes, you might need specialized expertise in a particular area, such as bioanalytics or data management. This is where specialized CROs come in.
Advantages
- Expertise: These CROs offer deep expertise in specific areas, making them the go-to choice for specialized projects.
- Quality: Specialized CROs often produce higher-quality data in their area of expertise, leading to more robust findings.
- Speed: With a focus on a specific aspect of drug development, specialized CROs can often complete tasks more quickly than their full-service counterparts.

The Role of CROs in Clinical Trials
Clinical trials are the make-or-break stage of drug development. It’s where a potential drug proves its efficacy and safety in human subjects. Given the high stakes, it’s no surprise that this is one of the most regulated aspects of the pharmaceutical industry.
Phases of Clinical Trials
Clinical trials are generally divided into four phases, each with its own set of objectives and methodologies. Here’s how CROs contribute to each:
- Phase I: This is the first time the drug is tested in humans. CROs help design the trial, recruit volunteers, and ensure all ethical guidelines are followed.
- Phase II: This phase tests the drug on a larger group of people to assess its efficacy and side effects. CROs manage the data collection and statistical analysis.
- Phase III: This is the final stage before the drug is submitted for regulatory approval. CROs often manage these large-scale, multi-site trials, ensuring they meet all regulatory requirements.
- Phase IV: Also known as post-market surveillance, this phase involves monitoring the drug’s long-term effects. CROs help design and manage these studies, collecting data that can lead to the drug being refined or even withdrawn from the market.
To learn more about API clinical trials, read EVERYTHING YOU NEED TO KNOW ABOUT API CLINICAL TRIALS.
Regulatory Compliance
Navigating the maze of regulations that govern clinical trials is no small feat. Different countries have different rules, and even within a country, regulations can vary by state or province. CROs are experts in regulatory compliance, ensuring that the trials they manage adhere to all local, national, and international guidelines.
Ethical Considerations
Beyond the legal requirements, clinical trials also have ethical considerations. CROs ensure that all trials are conducted with the participants. This involves obtaining informed consent, ensuring data privacy, and adhering to ethical standards like the Declaration of Helsinki, which sets ethical principles for medical researchers.
Quality Assurance
Quality assurance is another critical aspect of regulatory compliance. CROs implement rigorous quality control procedures to ensure that the data collected is accurate, reliable, and conducted in an ethical and scientific manner. This is crucial for the approval process and maintaining the research’s integrity.
Benefits and Challenges
Benefits
Working with a CRO comes with many benefits that can make the drug development process smoother and more efficient. Let’s delve into some of these advantages:
- Scalability: One of the significant benefits of working with a CRO is the ability to scale operations up or down based on project requirements without the hassle of hiring or firing in-house staff.
- Access to Latest Technology: CROs are at the forefront of technological advancements in drug development. By outsourcing, companies can take advantage of cutting-edge technologies without the capital expenditure.
- Strategic Partnership: Many CROs go beyond being mere service providers to become strategic partners, offering insights and expertise that can shape the direction of research and development efforts.
Challenges
While CROs offer numerous advantages, it’s essential to be aware of potential challenges:
- Lack of Control: When you outsource critical functions, you give up a certain level of control, which can concern some companies.
- Cultural and Language Barriers: If your CRO is based in a different country, cultural and language differences can pose challenges in communication and project management.
- Data Security: Handling sensitive data comes with the risk of data breaches or unauthorized access, so it’s crucial to vet the security protocols of a potential CRO carefully.
How to Choose a CRO
Expertise
The first thing to consider when choosing a CRO is their expertise in your specific therapeutic area. Look for case studies, publications, or references that demonstrate their competence in your field.
Certifications
Certifications like Good Manufacturing Practices (GMP), Certificate of Suitability (CEP), and Food and Drug Administration (FDA) approval are not just jargon; they’re indicators of a CRO’s commitment to quality and regulatory compliance.
Reputation
Word of mouth and client testimonials can provide valuable insights into a CRO’s reliability and quality of work. Don’t hesitate to ask for references or check online reviews.
Conclusion
Contract Research Services (CROs) are the unsung heroes of the pharmaceutical industry. They offer specialized services that streamline the drug development process, making it faster, more efficient, and more cost-effective. Whether you’re a pharmaceutical giant or a small biotech startup, understanding the role and benefits of CROs can offer valuable insights into how to bring your drug from the lab to the market successfully.