
 Show all images
Show all images
Lannett Company - CDMO/CMO
Founded in 1942, Lannett Company, Inc. has an extensive history of manufacturing various unique pharmaceutical indications including over 100 currently distributed products. As a long-established CDMO, we utilize our deep industry knowledge and provide our clients with ideal end-to-end solutions that meet their needs. Safety, quality, and productivity are of the utmost importance when choosing a manufacturing partner. Understanding this, our dedicated staff utilizes in-house talent and manufacturing capacities (3.5B Oral Solid doses and 2.0M liters of liquids per year) to ensure that these business objectives are met.

United States

Over 100 unique marketed pharmaceutical products and 17 approved product applications since 2019

Manufacturing capacities : 3.5B Oral Solid doses and 2.0M liters of liquids per year

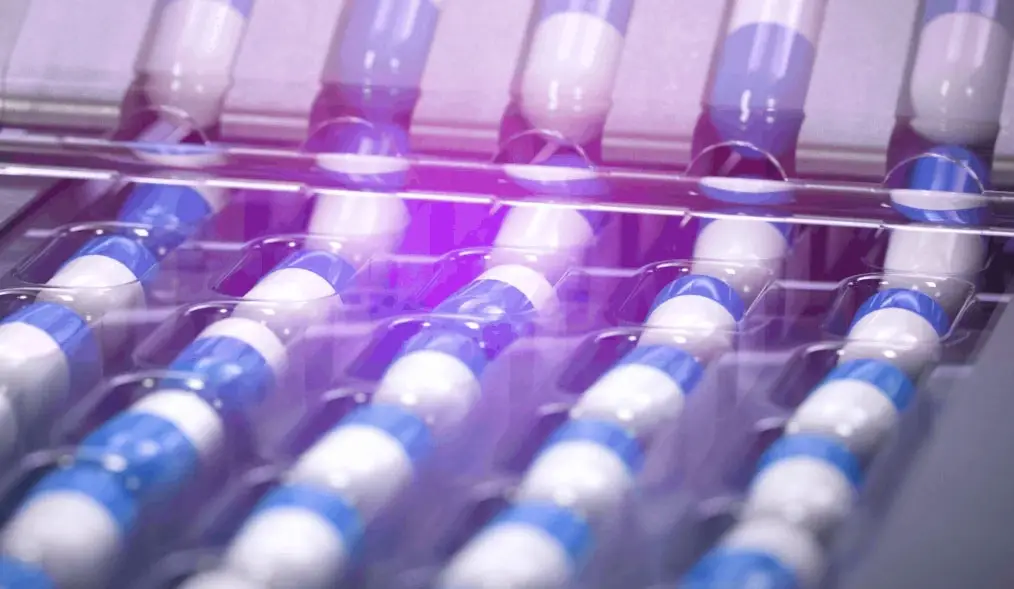
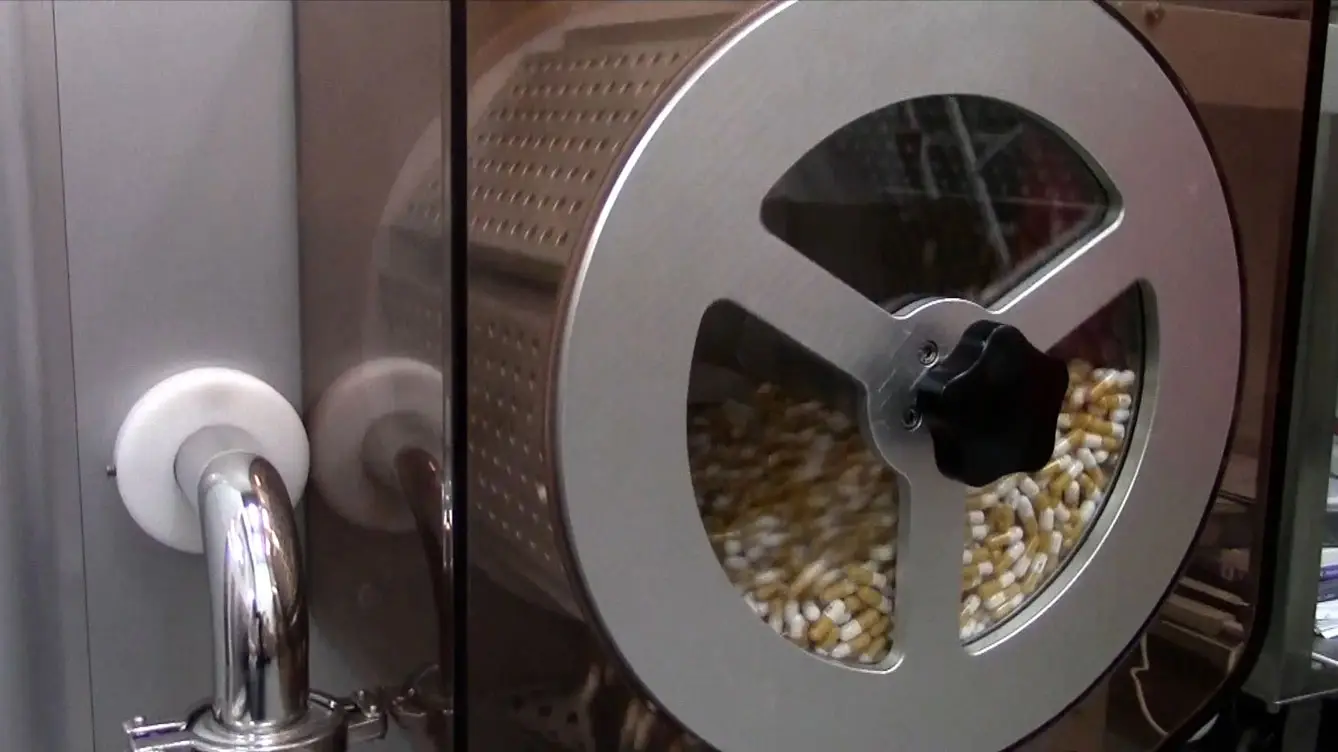
We specialize in contract manufacturing of Oral Solid Dose (OSD) and Liquids, including high potency substances and small molecules.
Our services:
Production scale:
Response time:
Services
Drug Product (CMO)
Replies slower than most
Replies slower than most
Replies slower than most
Replies slower than most
Contact Lannett Company for Contract Services Expertise
Connect with Lannett Company, a leading pharmaceutical company from United States. They offer specialized Drug Product (CMO), of which the services for CMO/CDMO include Tablets & Capsules, Powders & Granules. Contact Lannett Company for free and discover if they are the perfect partner for your pharmaceutical needs.
Drug Product (CMO)
Drug Product CMOs focus on the manufacturing and packaging of finished pharmaceutical dosage forms. These contract partners provide scalable production solutions that meet global regulatory and quality standards, supporting pharma companies throughout the drug product lifecycle.
Pharmaoffer’s Drug Product CMO category includes providers capable of manufacturing oral solids, injectables, topical formulations, and more—whether for clinical trials or commercial distribution.
Key Functions of Drug Product CMOs:
Formulation Development: Supporting the transition from drug substance to drug product through formulation optimization and compatibility testing.
Clinical and Commercial Manufacturing: Offering pilot-scale to high-volume manufacturing for various dosage forms under GMP conditions.
Packaging and Serialization: Providing primary and secondary packaging with serialization and tamper-proof labeling to meet compliance requirements.
Regulatory Support: Ensuring that all production meets FDA, EMA, and ICH standards for drug product quality, safety, and traceability.
Flexible Production Lines: Handling diverse dosage forms including tablets, softgels, liquids, and sterile injectables.
Partner with Proven Drug Product CMOs
Through Pharmaoffer, discover CMOs that excel in drug product manufacturing. Whether you need small-scale batches or full commercial output, our platform connects you with the right partners for compliant, cost-effective solutions.
Oral solid products
Oral solid products include tablets and capsules. CMOs provide formulation development, manufacturing, and packaging of solid dosage forms.
Tablets & Capsules
Tablets and capsules are common oral solid forms. CMOs offer formulation, compression, encapsulation, and GMP-compliant manufacturing services.
Powders & Granules
Powders and granules are flexible oral formats. CMOs support blending, granulation, and GMP filling for pharmaceutical applications.



 Follow us:
Follow us: