Small molecules vs. Biologics
Understanding the differences

Su Keles | Posted on October 11, 2022 | Edited on September 27, 2023
- Introduction
- Role of Small Molecules
- Rise of Biologics
- What Defines Small-Molecule Drugs?
- Understanding Biologics (large-molecule drugs)
- Method of Administration
- Economic Considerations
- Regulatory Landscape
- Biosimilars and Generics: An Important Distinction
- Patient-Centric Approach
- The Future Landscape
- Conclusion
Introduction
In a world where the pace of medical innovation never slows down, how do we keep up? Especially when it comes to understanding drug therapies, there are now more options than ever. From small molecules to biologics, these therapies offer a range of options to tackle diseases. But what distinguishes these two major classes of drugs? This comprehensive guide will delve into the similarities, differences, and applications of small molecules and biologics.
The Pharmaceutical Landscape
Role of Small Molecules
Small molecules have been the cornerstone of pharmacology for decades. They comprise a large segment of today’s prescription medications, providing solutions for common and chronic conditions.
Rise of Biologics
On the other hand, biologics are the rising stars, offering treatments for complex diseases like cancer and autoimmune disorders. But why is this shift occurring? Let’s unpack it.
What Defines Small-Molecule Drugs?
Chemical Composition
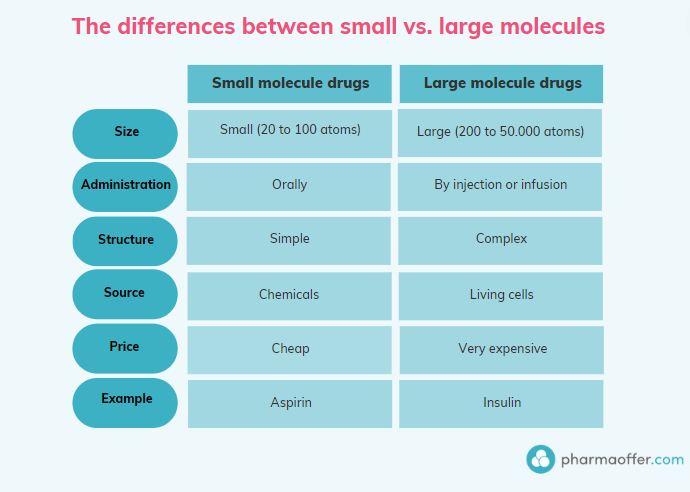
Small-molecule drugs are low-weight compounds, usually containing between 20 to 100 atoms. They’re synthesized chemically, making them stable and easier to manufacture.
Advantages and Challenges
Their small size and stable nature allow for easy administration, typically through oral routes. However, their simplicity can sometimes result in increased competition from generic drugs after the patent expires.
Understanding Biologics (large-molecule drugs)
Source and Composition
Biologics are made from living organisms, including humans, plants, and microorganisms. With complex structures that can contain up to 50,000 atoms, they’re inherently more challenging to develop and manufacture.
Key Benefits and Drawbacks
Biologics offer highly targeted treatments but come with limitations like higher costs and more complex administration methods.
Method of Administration
Oral vs. Injectable
While small molecules are often administered orally in pill or tablet form, biologics typically require injections due to their sensitivity and size. What’s the patient experience like for each?
Compliance Challenges
Adhering to a treatment regimen can be trickier with biologics. Their injection-based delivery methods might require more frequent visits to healthcare providers, affecting patient compliance.
Economic Considerations
Cost of Production
Small molecules are generally cheaper to produce. Biologics, on the other hand, involve complex manufacturing processes, driving up their cost.
Impact on Patient Wallet
Due to higher production costs, biologics are often more expensive for the end-user. This raises critical questions about accessibility and affordability.
Regulatory Landscape
Approval Pathways
Both drug classes are subject to rigorous approval processes, but biologics often require more detailed clinical trials due to their complexity. How does this impact time-to-market?
Intellectual Property Concerns
Patents and generics pose different challenges for small molecules and biologics, affecting market competition and drug availability.
Biosimilars and Generics: An Important Distinction
What are Biosimilars?
Biosimilars are akin to generic drugs for biologics, but there are key differences. Unlike generics, biosimilars aren’t exact replicas of the original drug, but they have to meet rigorous standards to be considered “highly similar.”
Implications for Affordability
Biosimilars offer a less expensive alternative to high-cost biologics, but how much can they really save you?
If you want to refresh your memory on generic medicine, check out our blog!
Patient-Centric Approach
Personalized Medicine
The advent of biologics has opened doors to more personalized treatments, especially in the realm of oncology and autoimmune disorders. How does this compare with small molecules?
Safety and Side Effects
Although both drug types undergo rigorous testing, biologics tend to have fewer side effects due to their targeted approach. But what happens when the body rejects a biologic?
The Future Landscape
Emerging Technologies
Advancements like CRISPR and mRNA vaccines are pushing the boundaries of what’s possible in drug development. How will these technologies influence the small molecule and biologic sectors?
Global Medicine Shortage
The role of small molecules and biologics in combating global medicine shortages can’t be overstated. What are the considerations for global access?
Conclusion
In the ever-evolving landscape of pharmaceuticals, both small molecules and biologics have distinct roles to play. While small molecules offer affordability and ease of administration, biologics provide targeted and often more effective treatments for complex diseases. The key is not to see one as better than the other but as complementary tools in our healthcare arsenal.
FAQ
What Are Small-Molecule Drugs?
Small-molecule drugs are chemical compounds with low molecular weight, usually between 20 to 100 atoms. They are easier to manufacture and often cheaper than their large-molecule counterparts. These drugs are usually administered orally and are more stable, making them popular for treating a variety of conditions.
What Are Biologics or Large-Molecule Drugs?
Biologics are complex drugs manufactured from living organisms like humans, plants, and microorganisms. These drugs contain between 200 to 50,000 atoms and are more challenging to manufacture than small-molecule drugs.
Are Biologics Safer Than Small Molecules?
Biologics generally have fewer side effects but may pose risks like immune responses.
What Are Biosimilars?
Biosimilars are alternatives to biologics that are highly similar but not identical. They offer a less expensive treatment option.






Check out all other blogs here!